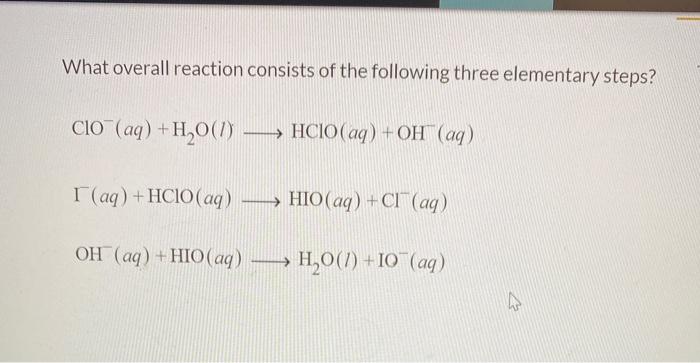
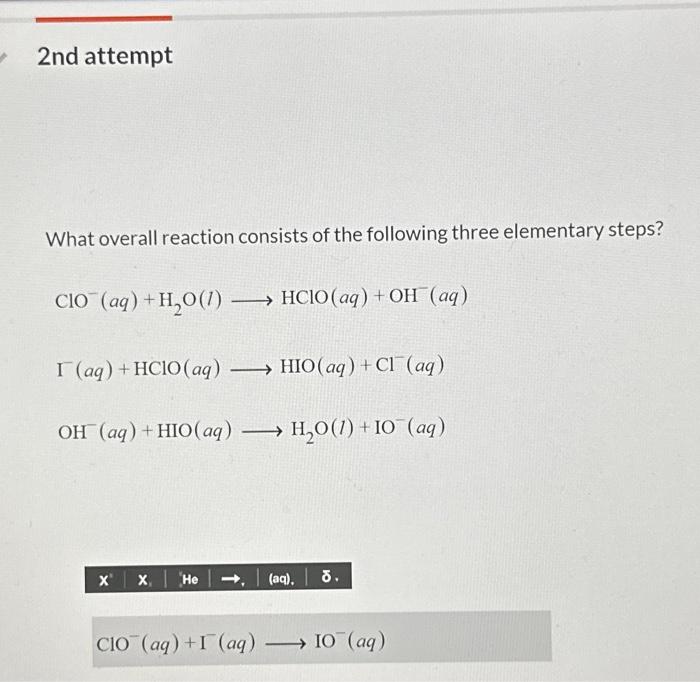
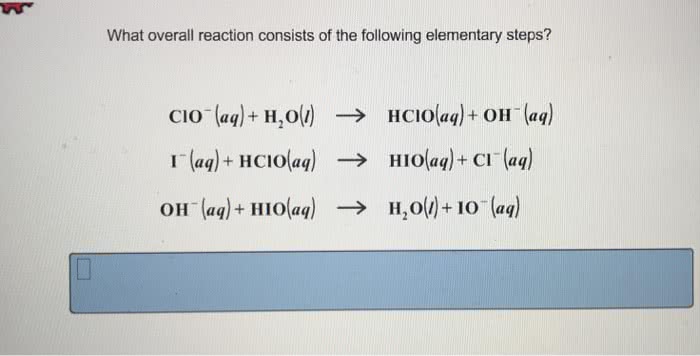
What overall reaction consists of the following three elementary steps? This question lies at the heart of understanding the intricate mechanisms that govern chemical reactions. Delving into the realm of elementary steps unveils a captivating tapestry of molecular interactions, revealing the fundamental building blocks of chemical transformations.
Elementary steps, the indivisible units of chemical reactions, provide a window into the dynamic choreography of atoms and molecules. By dissecting overall reactions into their constituent elementary steps, we gain invaluable insights into the intricate pathways that lead to the formation of new substances.
Overall Reaction and Elementary Steps
An overall reaction represents the net chemical change that occurs during a reaction. It involves the reactants, products, and the overall stoichiometry of the reaction. Elementary steps, on the other hand, are the individual, fundamental chemical reactions that make up the overall reaction.
They represent the smallest indivisible chemical changes that can occur.
For example, the combustion of methane (CH 4) with oxygen (O 2) to form carbon dioxide (CO 2) and water (H 2O) can be represented as the overall reaction:
CH 4+ 2O 2→ CO 2+ 2H 2O
This overall reaction can be broken down into a series of elementary steps, such as:
- CH 4+ O 2→ CH 3O 2
- CH 3O 2+ O 2→ CO 2+ H 2O 2
- H 2O 2→ H 2O + O
Characteristics of Elementary Steps
Elementary steps typically involve the interaction of a small number of molecules (typically one to three) and have a simple stoichiometry. They are often unimolecular (involving a single molecule) or bimolecular (involving two molecules). Elementary steps can be either exothermic (releasing energy) or endothermic (absorbing energy).
The reactants in an elementary step are the molecules that are consumed in the reaction, while the products are the molecules that are formed. Intermediates are molecules that are formed and consumed within the same elementary step.
Mechanism of Overall Reaction: What Overall Reaction Consists Of The Following Three Elementary Steps
The mechanism of an overall reaction describes the sequence of elementary steps that lead to the formation of the products. The mechanism must be consistent with the overall reaction and must account for the observed experimental data.
In the case of the combustion of methane, the mechanism involves the following steps:
- Initiation: A hydrogen atom is abstracted from methane by a radical species, such as OH or HO 2.
- Propagation: The hydrogen atom reacts with oxygen to form a hydroxyl radical (OH), which then reacts with methane to form CH 3O 2. CH 3O 2reacts with oxygen to form CO 2and H 2O 2. H 2O 2decomposes to form H 2O and O.
- Termination: The radicals react with each other to form stable molecules, such as H 2O and CO 2.
Kinetic and Thermodynamic Aspects
The kinetic and thermodynamic aspects of overall reactions and elementary steps play a crucial role in determining the rate and equilibrium of the reaction.
The rate of an elementary step is determined by its rate constant, which is a measure of the frequency of the reaction. The rate constant is influenced by factors such as temperature, pressure, and the concentration of the reactants.
The equilibrium constant of an overall reaction is determined by the equilibrium constants of the elementary steps. The equilibrium constant is a measure of the relative amounts of reactants and products at equilibrium.
Experimental Determination of Elementary Steps
The elementary steps of an overall reaction can be determined using a variety of experimental techniques.
One common technique is isotope labeling. In this technique, one or more of the reactants is replaced with an isotope of the same element. The isotope can be traced through the reaction, allowing the researchers to determine the order of the reaction and the identity of the intermediates.
Another common technique is kinetic analysis. In this technique, the rate of the reaction is measured as a function of the concentration of the reactants. The data can be used to determine the rate law for the reaction and the rate constants for the elementary steps.
Importance in Chemical Processes
Understanding the elementary steps of a chemical reaction is crucial for a variety of reasons.
In catalysis, the knowledge of elementary steps allows researchers to design catalysts that can accelerate the desired reaction while minimizing the formation of unwanted byproducts.
In reaction engineering, the knowledge of elementary steps allows researchers to design reactors that can maximize the yield of the desired product.
In environmental chemistry, the knowledge of elementary steps allows researchers to understand the mechanisms of pollutant formation and to develop strategies for reducing pollution.
Answers to Common Questions
What is the significance of elementary steps in chemical reactions?
Elementary steps provide a detailed understanding of the reaction mechanism, revealing the sequence of events that lead to the formation of products. They enable the determination of rate laws, equilibrium constants, and other kinetic and thermodynamic parameters.
How are elementary steps experimentally determined?
Experimental techniques such as isotope labeling, kinetic analysis, and spectroscopy are employed to identify and characterize elementary steps. These methods provide insights into the intermediates, transition states, and reaction pathways involved.
What is the relationship between elementary steps and the overall reaction rate?
The rate of an overall reaction is determined by the slowest elementary step, known as the rate-determining step. Understanding the elementary steps allows for the identification of the rate-determining step and the development of strategies to optimize reaction rates.